
Nov. 11, 2024
Welcome to IDEAL's ultimate guide to Anodizizng Finishing in CNC machining!
Our blog is a comprehensive resource for anyone interested in learning more about this versatile and affordable surface finish option.
Anodizing creates a thin coat on non-ferrous metal parts, enhancing mechanical properties like strength, hardness, corrosion, and wear resistance. It is an electrolytic process offering many color options and is popular in industries requiring performance and aesthetics. This article is a comprehensive guide to anodizing, its work principles, factors affecting this finish, the types of anodizing process, and practical applications. After reading it, you will understand the process and its uses in part manufacturing.
What is Anodizing?
Anodizing is a surface treatment method that produces a thin film with a thickness ranging from 0.5 to 150 µm. The thin film enhances the corrosion resistance, wear resistance, strength, and surface hardness of non-ferrous materials like aluminum.
The anodized layer or oxide layer is formed electrolytically. It differs from conventional electroplating, which deposits another metal on the substrate. Instead, anodizing creates a thin coat that is a part of the metal surface.
Which Materials Can Be Anodized?
The anodizing process is primarily for metals. However, not all metals are compatible with it. Aluminum is the most common of all the anodizing materials, hence the term anodizing aluminum. Titanium and magnesium are also very popular in several industries. Metals like copper and iron are incompatible with anodizing because the oxides formed are unstable and will flake off, exposing the substrate surface to further corrosion.

In addition, plastics are not compatible with the anodizing process. While some surface finish enthusiasts claim that conductive plastics like Polyetheretherketone with conductive fillers, Polyaniline, and Polypyrrole are compatible with anodizing, they are not. They are subjected to surface coating techniques different from the true anodizing process.
Below shows common materials that can be anodized, their properties, common uses, and why they are anodized.
Aluminum
Lightweight, naturally forms a protective oxide layer
Anodized easily, providing corrosion resistance and color options.
Titanium
-Strong, resistant to corrosion, biocompatible
-Anodizing enhances durability and allows for color variations.
Magnesium
-Lightweight, decent strength, prone to corrosion
-It improves its corrosion resistance and surface strength.
Zinc
-High resistance to corrosion, softer metal
-Anodized mainly for protection in industrial environments.
Tantalum
-Excellent corrosion resistance, compatible with the human body
-It helps protect against corrosion in demanding applications.
Niobium
-Changes color during anodizing, resistant to corrosion
-Primarily anodized for its ability to create vibrant color effects.
Why is Aluminum the Most Used for Anodizing?
Aluminum is the most used metal for anodizing because its inherent properties support this surface treatment process and make it popular in many industries.
The primary reason for the compatibility of aluminum with anodizing is its strong natural tendency to form an oxide layer on exposure to air. Anodizing takes advantage of this behavior, thickening the oxide layer in a controlled and uniform manner.

Another reason aluminum is the most used with anodizing is that the aluminum oxide layer is porous, encouraging the absorption of dyes and sealants. After anodizing aluminum, sealing the pores locks the dyes, a feature not seen in many other anodized metals.
What’s more, aluminum has good electrical conductivity, which allows for the effective passage of electric current during anodizing. As a result, the anodizing process becomes more efficient, creating a uniform oxide layer on the surface.
Anodizing Process: How Does it Work?
The anodizing process is an electrolytic with metals like aluminum as the substrate. The substrate is connected to the positive terminal (anode), and a highly conductive metal like stainless steel or aluminum is connected to the negative terminal (cathode).
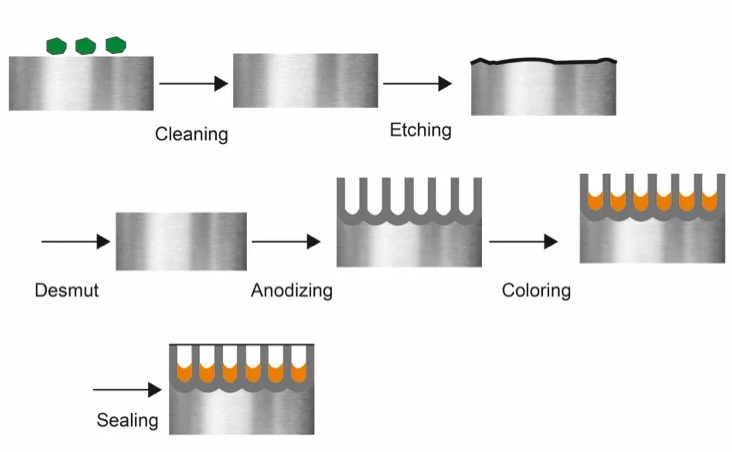
Preparation and Cleaning
Before anodizing a part, it must have a smooth and uniform surface. Mechanical surface treatment techniques like sanding, polishing, grinding, and bead blasting remove irregularities. Chemical cleaning techniques like alkaline or acid cleaning can remove contaminants like grease and oils. In addition, rinse with deionized water can remove residual cleaning agents.
Electrochemical Process
Make the workpiece the anode and a highly conductive metal like stainless steel or aluminum the cathode. Both are immersed in the electrolytic bath containing sulfuric or chromic acid and pass electricity through the electrolysis setup to cause the anode to oxidize and lose electrons.
Oxidation
Anodizing Bath and Reaction
Electrolytes used in anodizing can incorporate color into metal parts via dyeing, electrolytic coloring, and integral coloring.
In terms of dyeing, after anodizing, the oxide layer absorbs dyes. In electrolytic coloring, metal salts are deposited into the oxide layer’s pores through an electrochemical process, producing fade-resistant colors. Lastly, the integral coloring color is formed directly in the oxide layer, typically resulting in darker shades like bronze and black anodizing.
Sealing
You can seal the anodized layer using cold sealing, mid-temperature sealing, or hot sealing to reduce the formation of corrosion, scratches, and stains.
Cold sealing is the immersion of the metal parts in a solution containing nickel-fluoride at room temperature. It creates a sealed layer of fluoro-aluminate. Mid-temperature sealing involves immersing the unsealed anodized part in a metal salt at 60-80°C and sealing the pores with the metal salts.
Hot sealing involves immersing the metal salt near-boiling deionized water at 95 to 100°C. The pores swell and close, forming a dense layer sealing the part. If the sealing is poor or absent, the porous metal oxide layer accumulates dust and debris.
Main Factors to Affect Anodized Finish
The quality of the anodizing depends on several factors, which include:
Base Metal Composition
The base metal composition affects the quality of the anodized finish. For example, comparing aluminum alloys subjected to anodizing shows that pure aluminum of the 1100 series has a smooth, uniform finish. In contrast, aluminum alloys containing silicon have darker, uneven finishes.
Surface Preparation
Cleaning and polishing to remove contaminants will lead to a better fusion of the anodized layer with polished surfaces, giving different textures, like matte or bright finishes. In contrast, poor preparation can cause streaks or uneven color.

Electrolyte
The electrolyte solution can determine the anodized film thickness and appearance. High bath temperatures produce thinner coatings, while lower temperatures create thicker, more durable layers.
Electrical Current and Voltage
High-voltage anodizing produces a thick but rough film while anodizing at a low voltage produces a thin and smooth film. Additionally, current density impacts the adsorption of dyes.
Time in the Bath
A longer anodizing time will lead to thicker films, which improve corrosion resistance, hardness, wear resistance, etc. Moreover, it can cause increased roughness observed in hard anodizing.
Types of Anodizing
There are three main types of anodizing processes: Type I, Type II, and Type III anodizing. This section will summarize each type and the properties of the resulting anodized part.
Chromic Acid Anodize (Type I)
Type I or chromic acid anodizing creates a thin film. As a result, they are more suitable for decorative and functional purposes. After sealing, it mimics the performance of type II and III thin films.
Type I anodizing process has a limited color option, limited to grey or dark gray. It is suitable for thin coating and fatigue strength with applications in making aircraft components, military equipment, and precision instruments.
Sulfuric Acid Anodize (Type II)
Type II is the most common type of anodizing process. It uses sulphuric acid as the electrolyte, creating a thicker layer than type I. Additionally, this anodized finish has better corrosion resistance and wear resistance.
Type II anodizing process has numerous or limitless color options. It is suitable for aesthetics and functional purposes with applications in consumer electronics, automotive parts, etc.
Hard Anodize (Type III)
Type III produced an anodized finish with a thickness from 25 to 100 microns. It is the densest and strongest type of anodizing, making it suitable for parts used in harsh environments. Type III anodization method can use chromic, sulphuric, or oxalic acids as electrolytes.
The type III anodizing process also has limited color options, including darker shades of grey to black. It is suitable for heavy-duty wear and corrosion resistance and has applications in hydraulic cylinders, military vehicles, and marine hardware.
Anodizing II and Anodizing III Differences
Anodizing II and Anodizing III refer to different types of anodizing processes commonly used for aluminum and other metals. Here are the primary differences between them:

Thickness and Hardness
· Anodizing II (Type II anodizing): This process typically results in a thinner anodic coating compared to Type III. The thickness ranges from 1 to 25 microns.
· Anodizing III (Type III anodizing or hardcoat anodizing): This process creates a much thicker and harder anodic coating. The thickness can range from 25 to 100 microns, and it produces a significantly harder surface compared to Type II anodizing.
Surface Finish
· Type II anodizing generally results in a smoother, more decorative finish. It is often used for aesthetic purposes where corrosion resistance and moderate wear resistance are required.
· Type III anodizing produces a matte or satin-like finish due to its thicker coating. It is primarily chosen for applications where durability, abrasion resistance, and protection against wear and corrosion are critical.
Properties
· Type II anodizing provides moderate corrosion resistance, improved appearance, and some degree of wear resistance.
· Type III anodizing (hardcoat anodizing) offers significantly enhanced hardness, wear resistance, and excellent corrosion protection. It is suitable for applications requiring high durability and prolonged exposure to harsh environments.

Process Characteristics
· Both Type II and Type III anodizing processes involve similar initial steps of cleaning and preparation of the metal surface, followed by immersion in an electrolytic bath where anodic oxidation occurs. The main difference lies in the voltage and current density applied during the process, which determines the thickness and hardness of the resulting oxide layer.
Applications
· Type II anodizing is commonly used in architectural applications, consumer goods, and wherever a decorative finish with moderate protection is sufficient.
· Type III anodizing is preferred for industrial applications such as aerospace components, military equipment, automotive parts, and any application requiring superior wear resistance and durability.
In summary, the choice between Anodizing II (Type II) and Anodizing III (Type III or hardcoat anodizing) depends on the specific requirements of the application, including desired thickness, hardness, durability, and aesthetic considerations.
Practical Tips When Choosing the Anodizing Process for Your Parts
To choose an anodizing process for your projects or parts, you need to consider the following practical tips:
Consider the Application
The operating environment or function of the parts will largely determine the type of anodizing properties. When working with parts that will be used in harsh environments, such as marine or outdoor use, hard anodizing (Type III) offers better durability and corrosion resistance. For aesthetic purposes, a better option is Type I or II anodizing.
Prioritize Aesthetics
If the part’s aesthetic appearance is important, Type II or sulfuric acid anodizing allows for a wide variety of dye colors to enhance the look of your parts.
Evaluate Alloy Composition
Pure aluminum works best for anodizing. Alloys with high silicon or copper content may have uneven, darker finishes.
Assess Thickness Requirements
For thicker anodized metal, you can increase the duration of the anodizing process. This will also improve wear and corrosion resistance. However, it will make the surfaces rougher.
Plan for Sealing
Ensure the anodized layer is sealed properly, especially for parts exposed to weathering, to improve durability and prevent fading or discoloration.
Conclusion
At IDEAL, we excel in providing top-quality custom machining services tailored to meet your project needs. Our advanced technology and skilled team ensure your specifications are met with exceptional accuracy and efficiency.
Contact IDEAL today to see how we can assist with your next project!

Search Blog

Hey there, I'm Abby!
At IDEAL RAPID PRODUCTION, I'm a Project Management Expert in custom manufacturing field for more than 15 years. We offer cost-effective machining services from China. Ask for a quote for your ongoing or upcoming projects now!

3D Printing: Process, Pros & Cons, and Applications
Feb. 21, 2025

Vacuum Casting: Process, Pros & Cons, and Applications
Feb. 21, 2025


An Engineer’s Guide to Design for Manufacturing (DFM)
Feb. 20, 2025
GET IN TOUCH WITH US
Navigation
RESOURCE
Contact Us
Tel: 0755-36957776
E-mail: info@idealrp.com
Skype: +86 135 2877 3620
Whatsapp: +86 135 2877 3620
Add.: Shenghua Building, Songgang, Bao'an,Shenzhen 518105
Add.: Room 4, 16/F, Ho King Commercial Building, 2-16 Fa Yuen Street, Mong Kok, Kowloon, Hong Kong
